Describe Jj Thomson Experiment on the Discovery of Electrons
Thomson in 1904 and is known as the Plum pudding model or the Thomson model of the atom. If I have seen a little further it is by standing on the shoulders of G.
Explain The J J Thomson Experiment To Determine The Specific Charge Of Electron Sarthaks Econnect Largest Online Education Community
- This allowed him to calculate a charge to mass ratio for the electrons.

. But the original idea was different. Cathode ray tubes are sealed glass tubes from which most of the air has been evacuated. The original model of an atom based on the discovery of the electron was proposed by J.
Electrons are located in an electron cloud which is the area surrounding the nucleus of the atom. British physicist JJ. Discovery of the electron - key takeaways In 1897 JJ Thomson discovered the electron by using cathode-ray tubes.
Thomson began experimenting with cathode ray tubes. These negatively charged particles are an integral part of all atoms. JJ Thomsons experiment on cathode tubes eventually led to the discovery of the electron.
Thomson invented to test the theory that negative charges in. Joseph John Thomson or JJ. He gave the proof of electrons in the atom by his experiment in which he had used cathode ray tubesThese tubes are a source of cathode rays production.
Thomson was awarded the 1906 Nobel Prize in Physics for the discovery of the electron and for his work on the conduction of electricity in gases. Cathode rays are nothing but a stream of negatively charged particles called electrons. Each generation benefits from the insights and discoveries of those who came before.
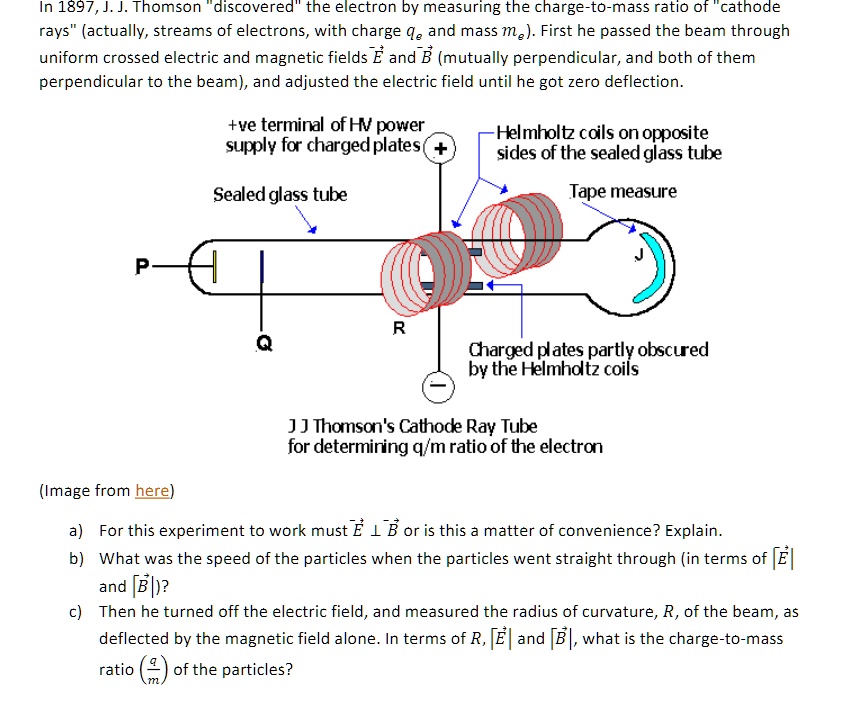
The gold foil experiment is one of the most important experiments. Thomson and the discovery of the electron. The speed of electron v 102 x 10 7 ms Radius of circular path r 6 cm 6 x 10 -2 m Magnetic induction B 10 -2 Wbm 2.
In 1897 Thomson showed that cathode rays were composed of previously unknown negatively charged particles which he calculated must have bodies much smaller than atoms and a very large. Thomson studied the characteristics and the constituents of cathode rays. In the late century physicist JJ.
J Thomson 18561940 performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube an area being investigated by many scientists at the time. Thomson who in 1897 showed the existence of the charged particles that came to be known as electrons. Three big experiments that helped shape the electron and the atom concept that we have today are the discovery of the.
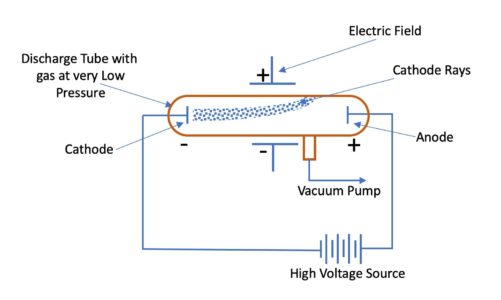
Both subjects were transformed by the experiments of J. The glass tube is attached to a vacuum pump and the pressure inside the tube is reduced to 001mm. Experiments with cathode ray tubes by Thomson showed that all the atoms contain tiny subatomic particles or electrons that are negatively charged.
The scientist named JJ. Electrons were the first of sub-atomic particles to be discovered by JJ. A cathode ray tube consists of two thin pieces of metals called electrodes sealed inside a glass tube with sealed ends.
Thomson suggested the atoms plum pudding concept which had negatively charged electrons trapped in a soup filled with positive energy. Thomson made a detailed study of the discharge of electricity through gases under very low pressure. What is the cathode ray tube.
Thomson led to the discovery of negatively charged particles called electron. Cathode ray tubes are essentially sealed vacuum chambers made up of glass. Thomson built a cathode ray tube by putting two cylinders together and sending a voltage through them.
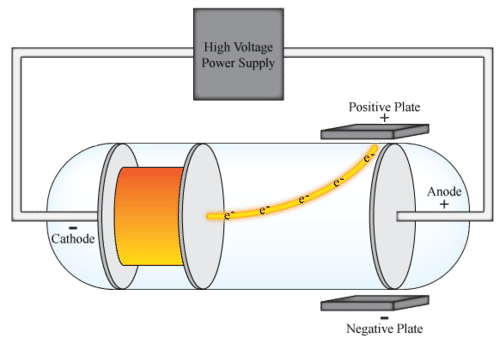
From his experiment Thomson arrived at the conclusion that. Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of bodies. Up to 24 cash back Thompson designed an experiment using magnetism to move the electrons in the opposite direction caused by the electric field.
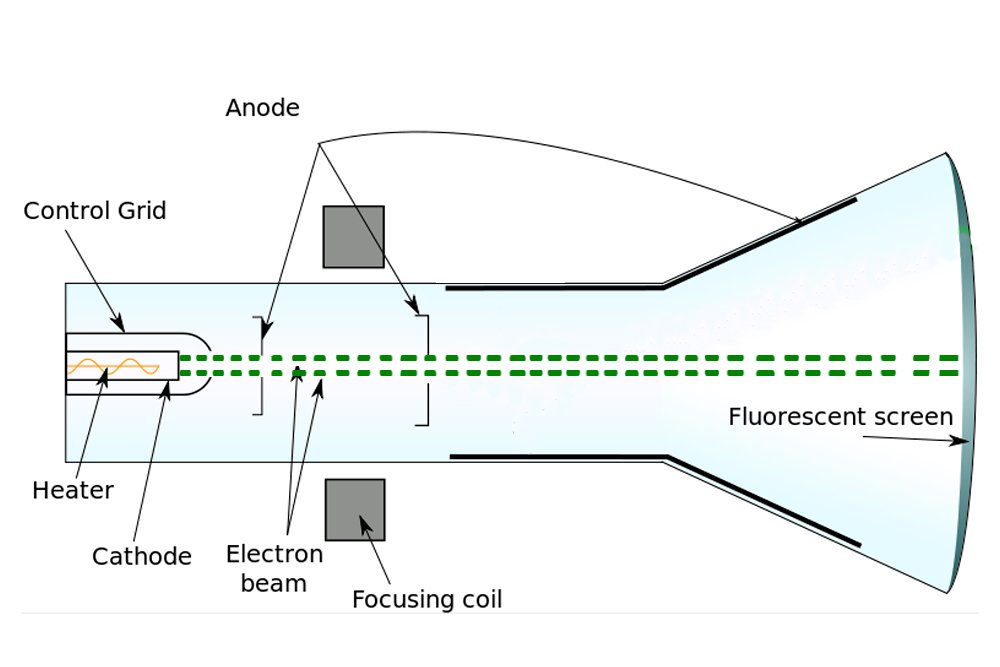
He did this using a cathode ray tube or CRT. The cathode ray discharge tube experiment performed by JJ. Cathode ray tubes are the glass tubes in which air is absent and in order to maintain this sealing is done at the ends.
Thomson discovered the electrons by cathode ray experiment. Sir Joseph John Thomson OM PRS was a British physicist and Nobel Laureate in Physics credited with the discovery of the electron the first subatomic particle to be discovered. It is a vacuum sealed tube with a cathode and anode on one side.
This experiment was performed using a cathode ray tube Crookes tube. Thomsons cathode ray experiment was a set of three experiments that assisted in discovering electrons. Thomson announces the discovery of electrons On April 30 1897 British physicist JJ.
Previously atoms were known to be indivisible but in 1897 J. In Thomsons experiment a beam of electrons travelling at 102 x 107 ms is bent into a circular path of radius 6 cm in a magnetic field of induction 10-2 Wbm2 normal to its path. Thomson the Discovery of the Electron and the Study of Atomic StructureOverviewLate in the nineteenth century physicists were working hard to understand the properties of electricity and the nature of matter.
Find the specific charge em. Thomson a British physicist conducted the cathode ray experiment. It consists of a glass tube connected to two metal electrodes at two ends.
Thomson announced his discovery that atoms were made up of smaller components. The cathode ray tube was what JJ. He zapped atoms with.
The British physicist Joseph John J. When a high voltage is applied across the electrodes placed on the ends of the tube a beam flows from the negatively charged pole to the positively charged pole. Thomson 1856 1940 On April 30 1897 English physicist Joseph John Thomson gave the first experimental proof of the electron which had been already theoretically predicted by Johnstone Stoney.
Thomson is the scientist that discovered electrons through an experiment called the Cathode Ray Experiment. Well he discovered electrons.
Who Discovered Electrons The Cathode Ray Experiment Selftution

Describe J J Thomson S Cathode Ray Tube Experiment And Explain How The Experiment Helped Add To Our Understanding Of The Atom Study Com

Atom Discovery Of Electrons Britannica

Who Discovered Electrons The Cathode Ray Experiment Selftution

Cathode Ray Experiment By Jj Thomson Crt Explanation Uses Of Cathode Ray Tube
Thomson S Atomic Model Ck 12 Foundation
Solved In 1897 J J Thomson Discovered The Electron By Measuring The Charge Mass Ratio Of Cathode Rays Actually Streams Of Electrons With Charge Qe And Mass Mg First He Passed The Beam Through Uniform

Q2 With Reference To The Disco Lido

Cathode Ray Tube Experiments By J J Thomson Chemistrygod
What Is Jj Thomson S Plum Pudding Model
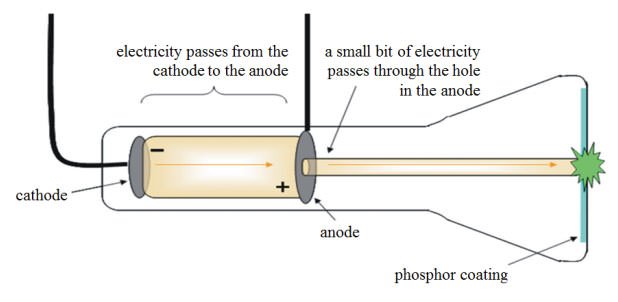
10 Major Contributions Of J J Thomson Learnodo Newtonic
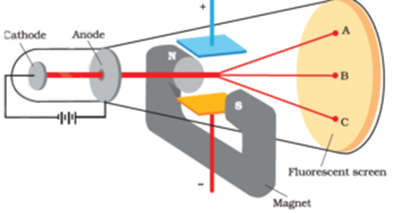
Discovery Of Electron Thomson S Experiment Milikan S Experiment
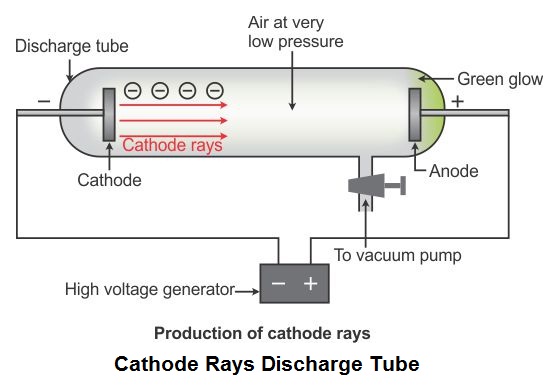
Describe J J Thomson 39 S Cathode Ray Experiment And How It Led To The Discovery Of Electrons What Are The Properties Of Electrons Chemistry Topperlearning Com N3yjj99
How Did Jj Thomson Discover The E M Ratio How Did Milikan Find The Charge Of Electron By Speeding And Slowing Down The Rate Of Fall Of The Charged Oils Quora
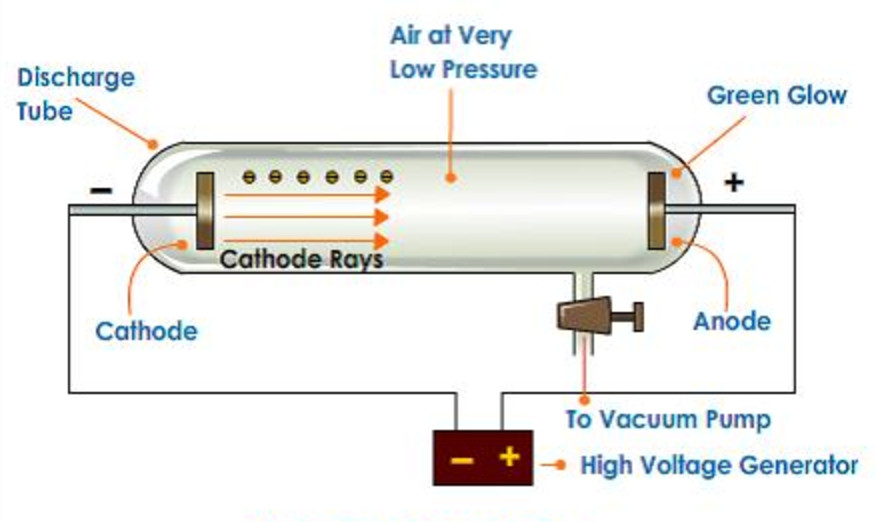
Discovery Of Electron Class 9 Structure Of An Atom

Describe J J Thomson S Experiment On The Discovery Of Electrons Brainly In

Evolution Of Atomic Theory Chemistry For Majors

Describe J J Thomsons Experiment On The Discovery Of Cathode Rays

Comments
Post a Comment